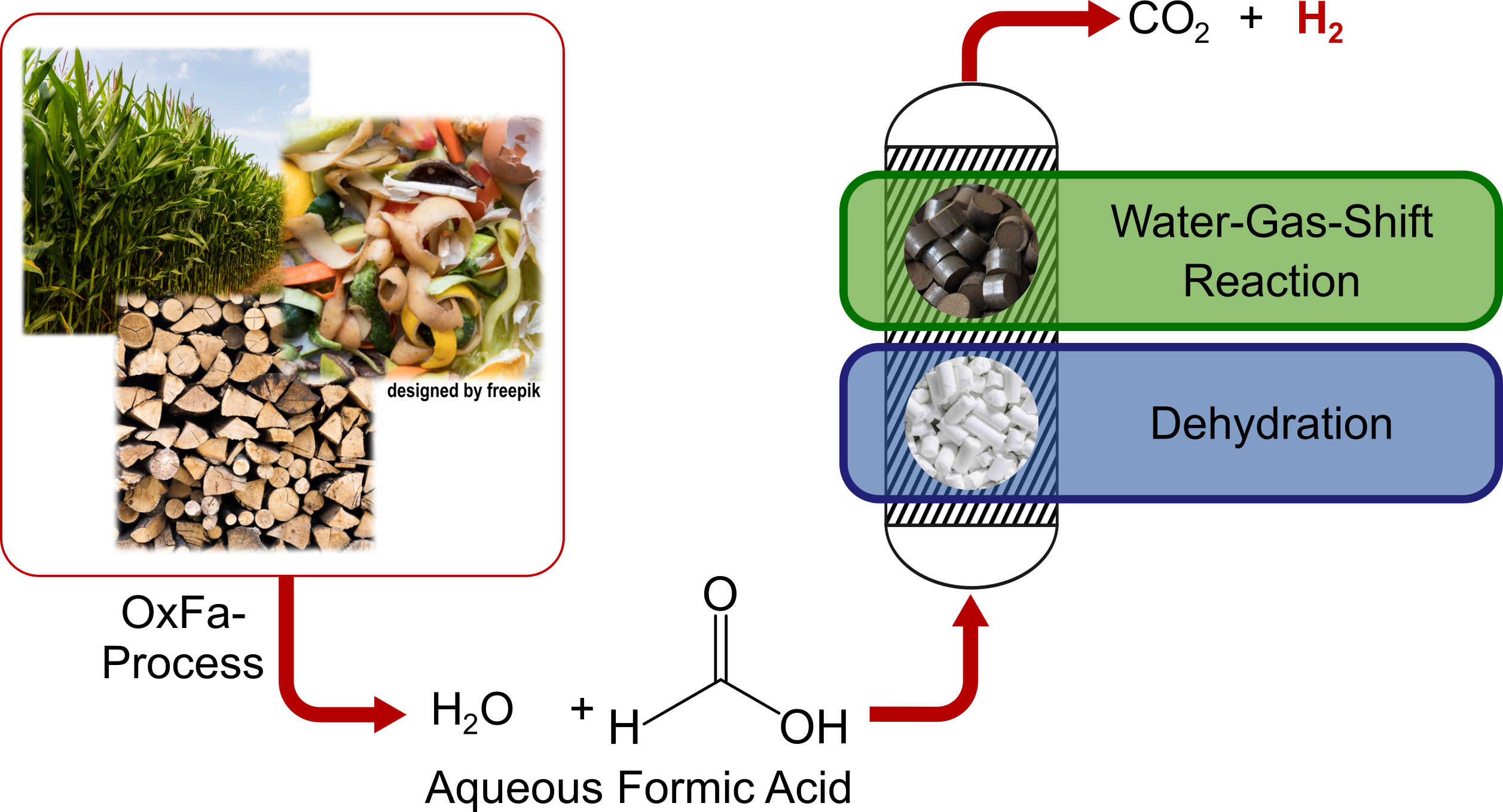
An alternative approach to the production of hydrogen from formic acid involves the development of a two-step process. In the first step, formic acid is dehydrated in the gas phase using acidic catalysts. The carbon monoxide (CO) and water (H2O) produced in this step are subsequently converted into carbon dioxide (CO2) and hydrogen (H2) through the water-gas shift (WGS) reaction.
The dehydration of formic acid is carried out using Brønsted acidic catalysts. Compared to dehydrogenation, significantly more cost-effective heterogeneous catalyst materials such as acidic zeolites, ion exchange resins or metal phosphates are suitable for this process. The dehydration process is considerably more stable than dehydrogenation because CO poisoning of the acidic catalysts is minimal. However, catalyst deactivation can occur over extended periods due to coking, which can be addressed by developing new catalysts with adjustable acidity.
The aim is to perform dehydration at high conversions to create optimal conditions for the WGS reaction and to protect the subsequent WGS catalyst from contact with formic acid. Due to the thermodynamic equilibrium limitations and the exothermic nature of the WGS reaction, this step should be conducted at temperatures between 100-200 °C to reduce the CO concentration to the ppm range. Novel catalyst systems for low-temperature WGS are being tested to achieve this. The high activity of these catalysts allows for very low CO formation during the WGS reaction, facilitating the purification of hydrogen to fuel cell quality.