With the transition of energy supply to renewable sources, hydrogen will play a crucial role due to its versatility. It will be used as a fuel (similar to natural gas), an energy storage medium, and a raw material for the production of various chemicals. Only if hydrogen is produced with low CO2 emissions, e.g., through wind, solar, or hydroelectric power, can its use contribute to achieving decarbonization. Since the production site of green hydrogen is often far from the consumer, it must be transported as efficiently and cost-effectively as possible. For this purpose, so-called chemical hydrogen carriers can be used, which bind the hydrogen and then release it again at the consumer’s location.
One such transport molecule is dimethyl ether (DME), which can be produced from carbon dioxide (CO2) and hydrogen (H2). It can be transported to a consumer by ship, where the hydrogen is released through steam reforming (Figure 1).[1]
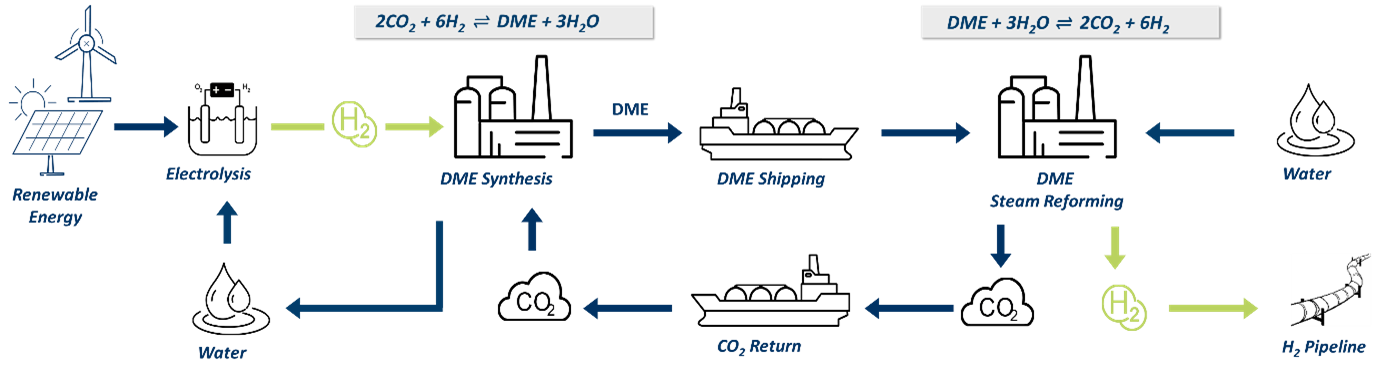
A significant advantage of this concept is the similarity in the physical-chemical properties of CO2 and DME, which allows for transport in the same ship. Additionally, during the synthesis of DME (typically in energy-rich, warm regions), water is produced, which can be used in electrolysis. At the location where the hydrogen is to be released, the water is reintroduced. This has the further advantage that only 50 mol% of the hydrogen needs to be transported via DME, with the other half coming from the water on-site. An initial economic assessment has shown that this concept can compete with other potential chemical hydrogen carriers (methanol, ammonia) due to its higher hydrogen capacity, higher gravimetric energy density, and lower toxicity.[1]
[1] Schühle et al., Dimethyl ether/CO2 – a hitherto underestimated H2 storage cycle, Energy Environ. Sci., 2023
