Dimethyl ether is a promising chemical carrier for long-distance hydrogen transport. The hydrogen is released at the consumer’s end via catalytic steam reforming. Initially, DME reacts with water through hydrolysis to form methanol.
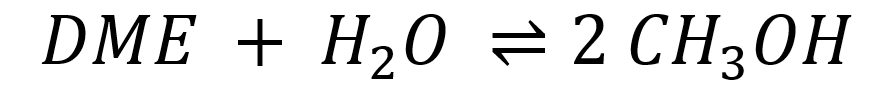
Subsequently, the resulting methanol is converted through steam reforming into carbon dioxide (CO2) and hydrogen (H2), resulting in the net reaction equation.
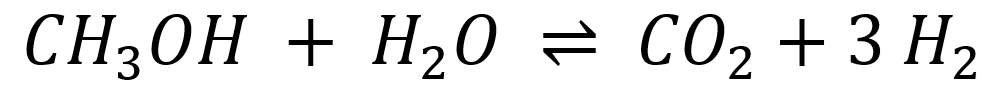
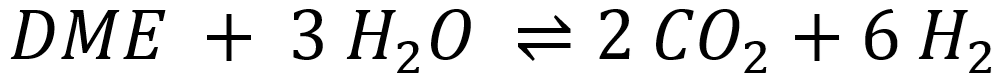
Two different catalytic functions are required to catalyze both reactions: acidic centers for the DME hydrolysis and metallic centers for the methanol steam reforming (MSR). For the first reaction, the choice of catalyst depends on the operating temperature. For reaction temperatures between 200 – 300 °C, zeolites with Brønsted acidic centers are typically used, whereas γ-alumina is generally employed for temperatures above 300 °C.
Of greater interest is the development of new MSR catalysts for the second reaction step, as the known catalysts (mostly copper-based) deactivate over time due to altered operating conditions (temperature, water partial pressure). Additionally, some catalysts promote side reactions, such as the formation of carbon monoxide (CO), which also leads to deactivation through coking. Due to these conditions, very few long-term stable catalysts with high hydrogen yields are known to date. Various catalyst systems are being investigated in a fixed-bed reactor (Figure 1) within the research group.
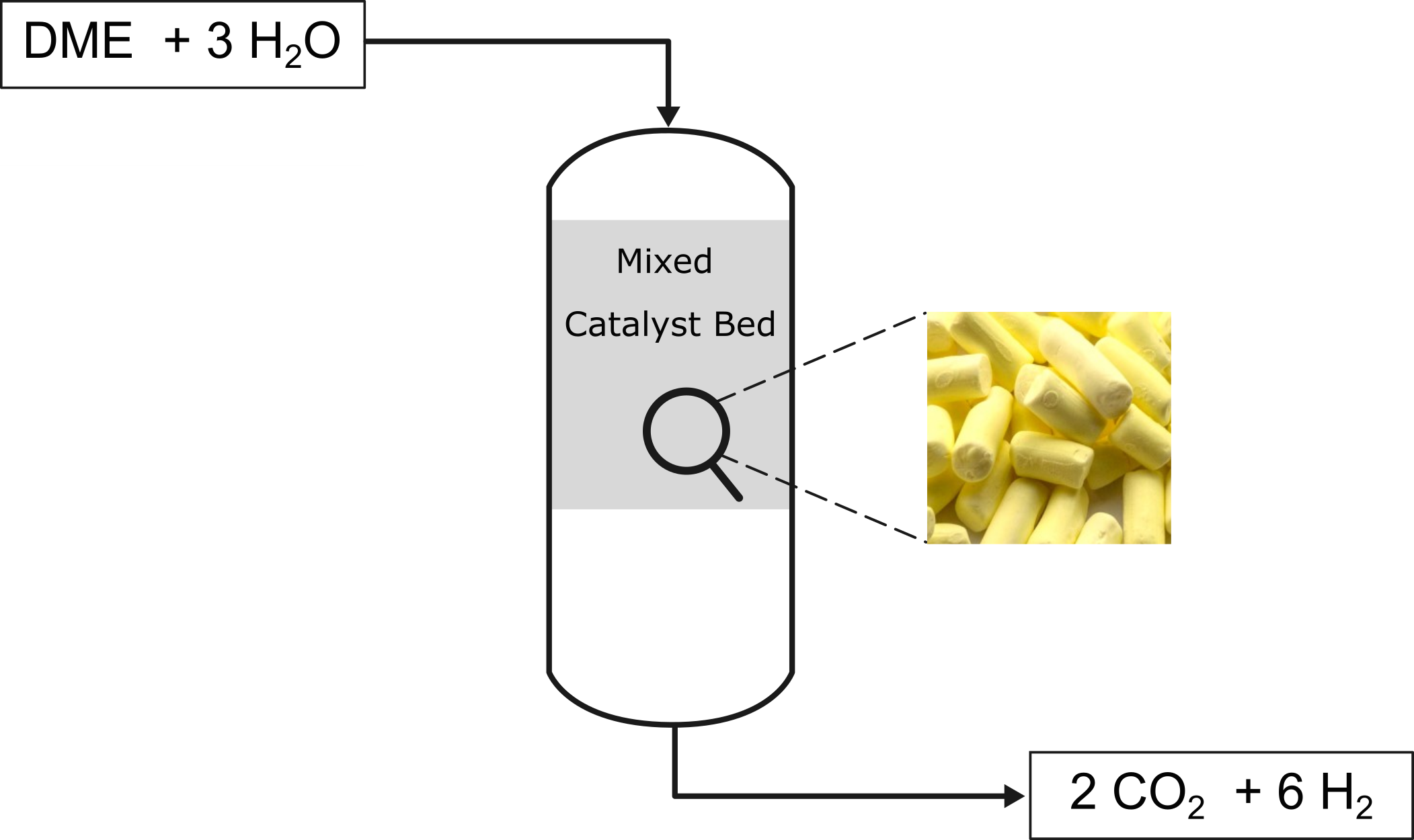
A particular focus is placed on indium-based catalysts, which have shown high selectivity and long-term stability for the second reaction step.[1]
[1] Stöber et al., A highly durable catalyst system for hydrogen production from dimethyl ether, Sustain Energy Fuels, 2024
