Fractionation and selective oxidation of lignocellulosic biomass to formic acid and high-grade cellulose
Lignocellulosic biomass is one of the most important renewable resources for the sustainable production of biofuels, bio based materials and platform chemicals. Lignocellulosic biomass is generated by atmospheric CO2, water and sunlight via photosynthesis and can therefore be regarded as a promising alternative to fossil resources with zero netto CO2 emissions that can be provided sustainably in large quantities. However, gaining value from lignocellulose is more challenging due to the higher complexity of the raw material and its higher recalcitrance towards selective processing. It typically consists of hemicellulose (25 %), lignin (25 %), cellulose (40 %) and ca 10 % other, minor components.
Cellulose is an attractive product for material applications like paper, while hemicellulose and lignin can be used for energy generation or the production of bulk chemicals. Due to the higher value of cellulose compared to hemicellulose and lignin, there are several approaches for fractionation of lignocellulosic biomass into its main components. This fractionation facilitates the selective use of cellulose for the paper industry and the further processing of lignin and hemicellulose for the production of bulk chemicals.
Formic acid (FA) is an important bulk chemical that is widely used in chemical, leather, pharmaceutical, rubber and other industries. Furthermore, FA can be easily and selectively decomposed to hydrogen and CO2 under mild reaction conditions. Hence, FA can be regarded as an attractive hydrogen storage material.
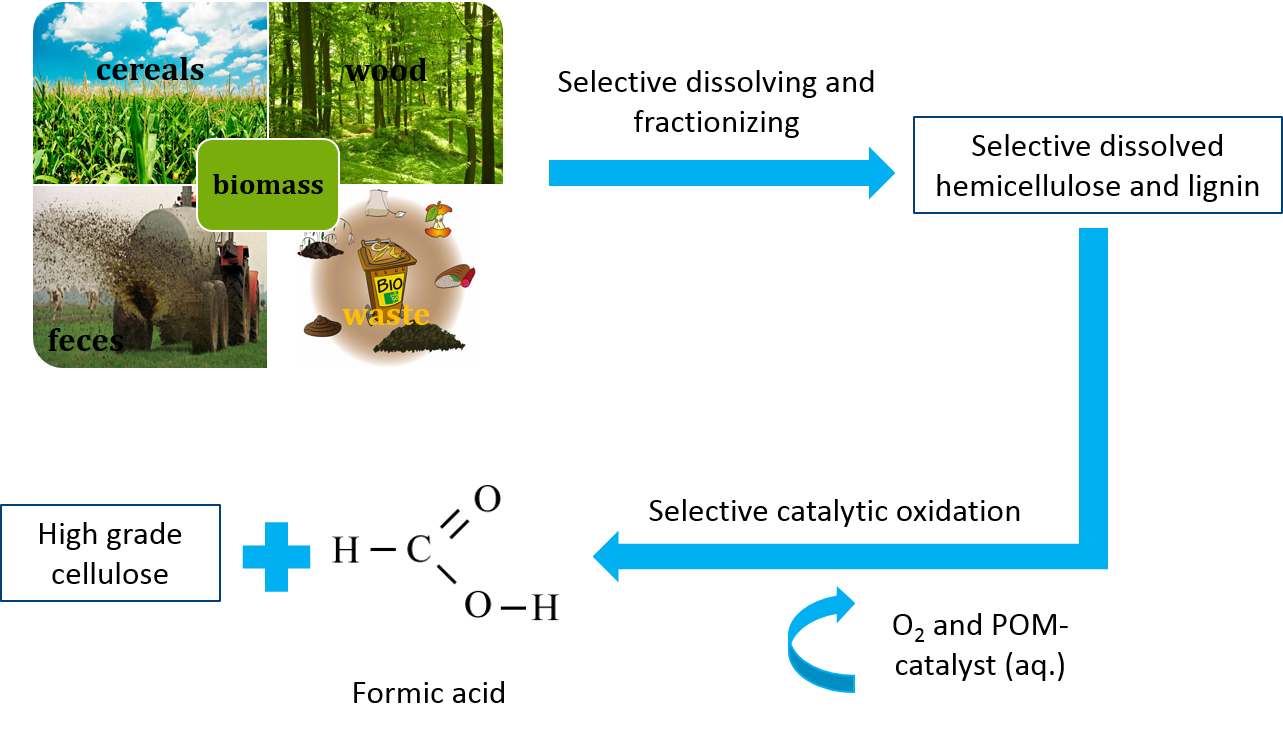
In this context, our working group investigates the fractionation of lignocellulosic biomass and selective in-situ conversion of hemicellulose and lignin to formic acid while cellulose remains untapped for further processing. For these approaches, several tailored polyoxometalate-catalysts as well as liquid reaction matrixes are used. Hereby, we collaborate with several partners from academia (Imperial College London) as well as industry (Chrysalix, UK and OxFA GmbH, DE).