Influence of N- and O-containing heteroatoms on the continuous oxidative desulfurization of liquid fuels
State of the Art
Based on the current debate on pollutant emission, stricter environmental regulations for the combustion of liquid fuels are being adopted. Also for the nitrogen oxide and fine dust emissions of motor vehicles the environmental regulations became stricter. On the one hand, higher combustion temperatures have to be achieved in order to reduce particulate matter, but on the other hand, it produces more NOx. In particular, to observe the NO2 limit of 34 ppbw in the air, a nearly complete removal of nitrogen from liquid fuels is essential.
The currently used technology for nitrogen removal is based on the hydrogenation of nitrogen compounds using NiMo- or CoMo- fixed bed catalysts (Hydrodenitrification, HDN).
Temperatures of 300 °C – 400 °C and hydrogen pressures of 40 bar – 170 bar are necessary for the HDN. These harsh reaction conditions are needed to hydrogenate even the difficult to remove N‑compounds such as pyridine, pyrrole and their derivatives. Since these harsh conditions and huge amounts of hydrogen are undesirable (note that 90 % of the hydrogen produced is currently obtained by steam reforming of fossil fuels!), there is a big interest in efficient, alternative hydrogen-free denitrification methods.
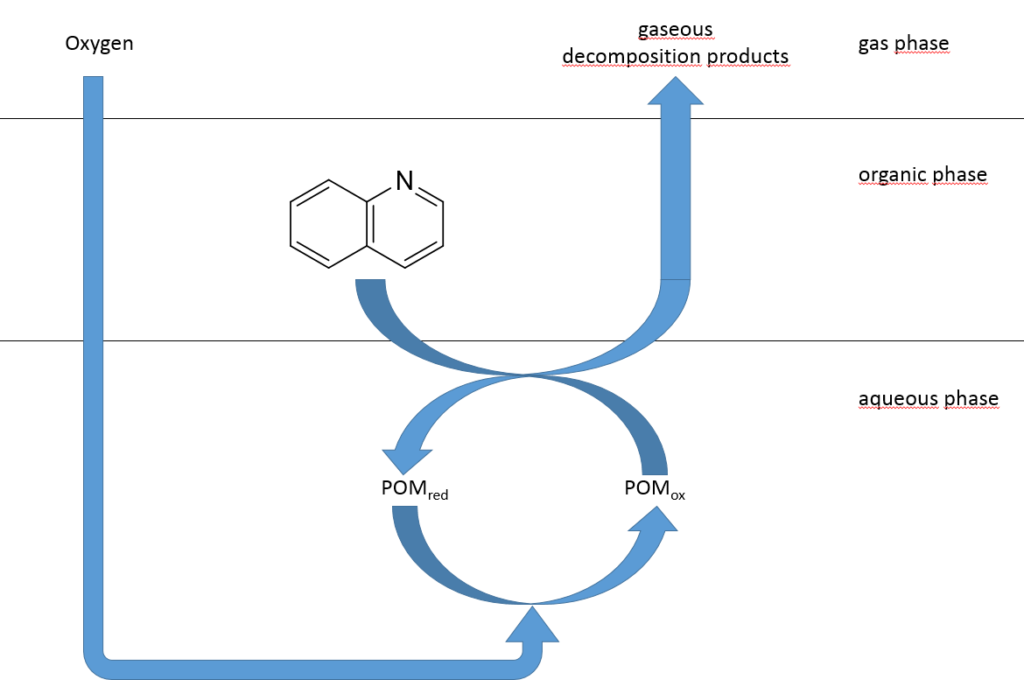
Oxidative Denitrification
An alternative to the expensive HDN is the oxidative denitrification (ODN). The organic nitrogen compounds are oxidized by means of a suitable oxidizing agent and the corresponding catalyst to CO2 and N2. Catalyst systems suitable for this purpose are polyoxometalates. Typical reaction temperatures for ODN are 30 °C – 120 °C and thus significantly lower than for the HDN. Hydrogen peroxide is commonly used as an oxidant, but initial experiments with elemental oxygen show promising results, which provides a cheaper and greener alternative.